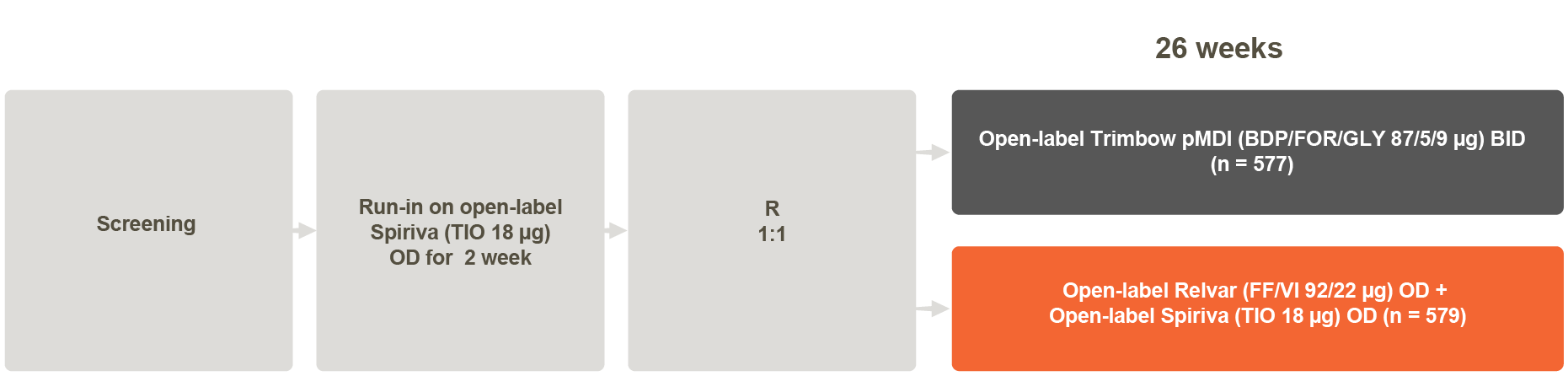
Study Design1
Endpoints1
Primary endpoint:
- Change in baseline SGRQ total score at Week 26
(Non-inferiority analysis: Trimbow vs Relvar + Spiriva)
Secondary endpoints included:
- SGRQ response (change from baseline in total score ≤-4) at Week 26
- FEV1 at Week 26
Population2
- Male or female ≥40 years
- COPD diagnosis ≥12 months prior to screening
- FEV1, <50% + FEV1/FVC ratio <0.7
- Current or ex-smokers with a history of ≥10 pack years
- ≥1 exacerbation in the 12 months prior to screening
- Symptomatic patient at screening with a CAT score ≥10
- Dual therapy (ICS/LABA, LAMA/LABA, ICS+LAMA), or LAMA monotherapy, for at least 2 months prior to screening
- Patients receiving triple therapy were excluded
Results1
TRISTAR met its primary endpoint of non-inferiority in mean change in SGRQ total score with Trimbow vs. Relvar + Spiriva; of note, pre-specified non-inferiority margins chosen were wide, and Relvar + Spiriva had numerically greater outcomes.
Mean change in SGRQ from baseline
At week 26, overall mean change in SGRQ total score from baseline was numerically greater with Relvar + Spiriva (overall mean difference: 1.04 units; 95% CI: -0.56, 2.65; p=0.204)

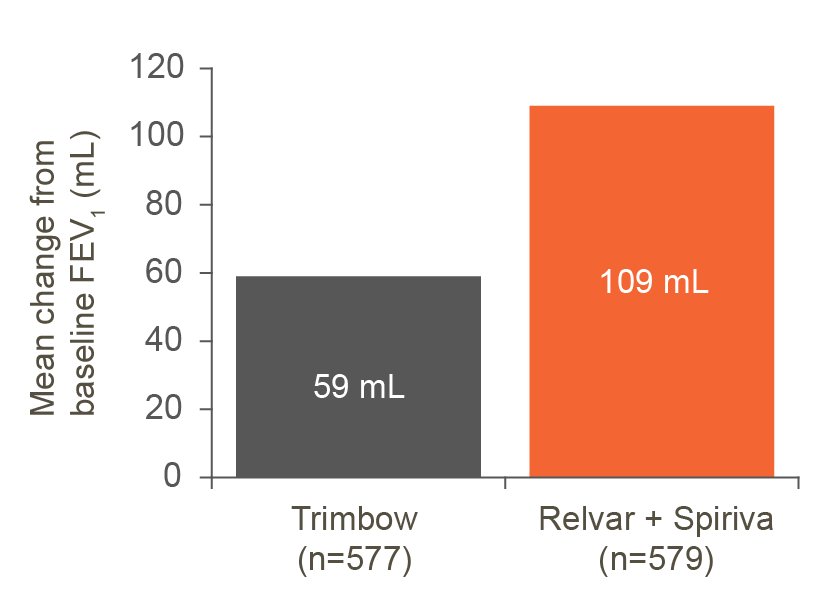
Mean change in FEV1 from baseline
Adjusted mean change in FEV1 was greater with the Relvar and Spiriva treatment arm vs. the Trimbow arm (50ml; 95% CI: NR; p=NR). No statistical analysis for this end point
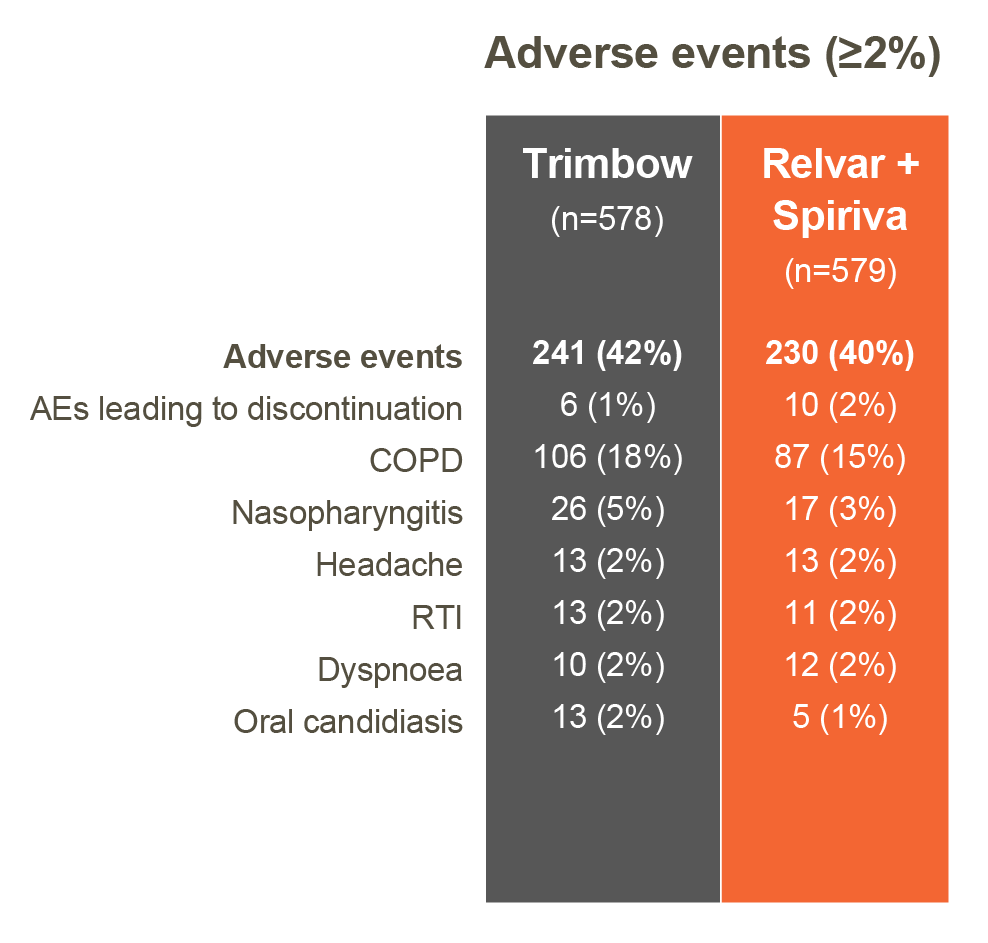
Safety1
TRISTAR: Trimbow and Relvar + Spiriva showed comparable safety profiles 1